/TC_608336-examples-of-physical-changes-5aa986371f4e1300371ebebb.png)
10 Examples of Physical Changes
A physical change involves changes in physical properties, but not chemical properties. For example, physical properties change during tempering steel, crystallization, and melting. Here are examples of physical changes: Crumpling a sheet of aluminum foil Melting an ice cube
/physical-and-chemical-changes-examples-608338_FINAL-f4e256e7fbf54f46a8c7bcefb300f5db.png)
Examples of Physical Changes and Chemical Changes
Change is happening all around us all of the time. Just as chemists have classified elements and compounds, they have also classified types of changes. Changes are either classified as physical or. 3.6: Changes in Matter - Physical and Chemical Changes - Chemistry LibreTexts Skip to main content Table of Contentsmenu

PPT Physical and Chemical Changes PowerPoint Presentation, free
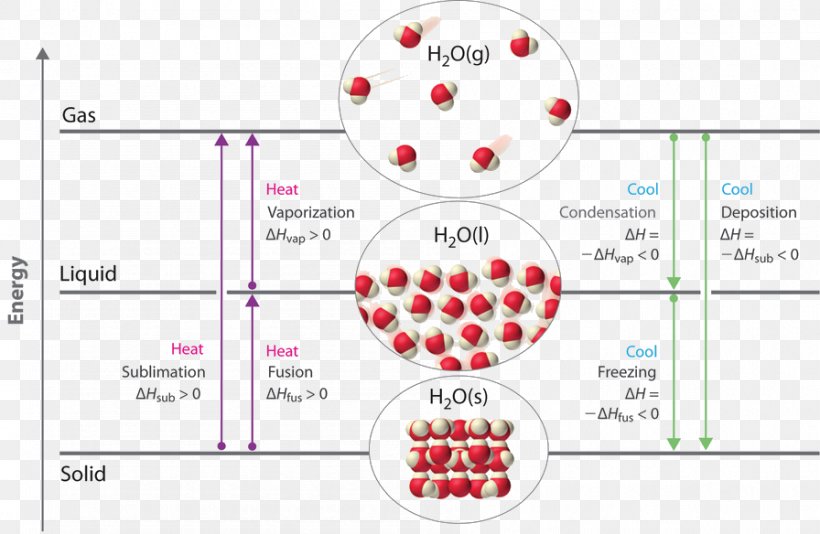
Solution: Physical: boiling and melting are physical changes. When water boils no bonds are broken or formed. The change could be written: H2O(l) → H2O(g) H 2 O ( l) → H 2 O ( g) Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Color changes indicate chemical change.
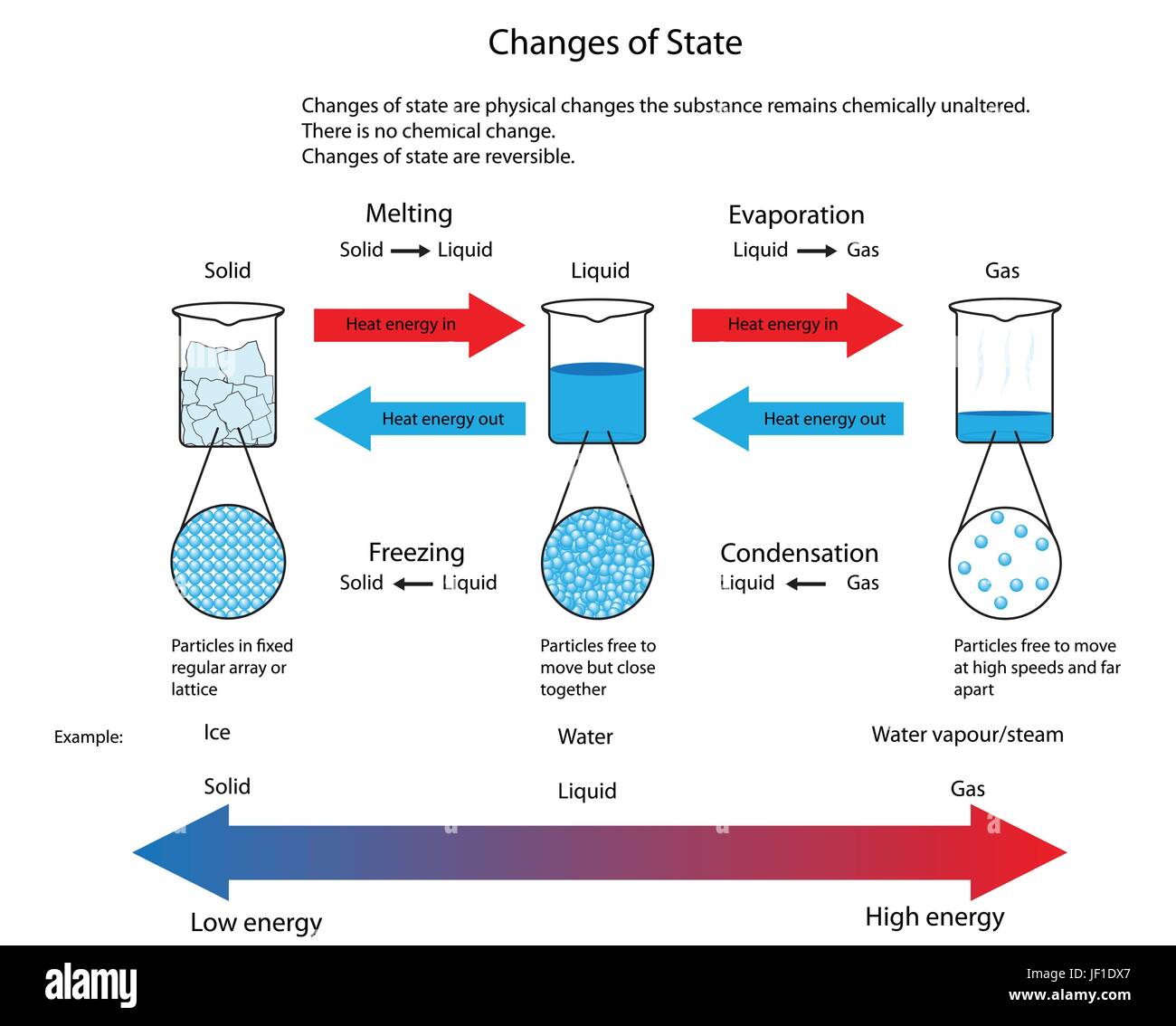
Diagram illustrating the physical changes of state from solid to liquid
physical change Definition change in size, state, or shape (the three S's) + 1 more side Term chemical change Definition results in the formation of one or more new substances + 1 more side examples of physical properties color, odor, hardness and texture, melting point, boiling point, electrical conductivity and magnetism

Physical Change Examples
Physical Changes. Physical changes are usually intermolecular changes (literally meaning "between molecules"), such as phase changes. Some examples are freezing water and cutting paper. The ice's molecules maintain the same atomic structure (H2O), but more hydrogen bonds between each water molecule are formed. Conversely, the paper's.

Chemical and Physical Changes of Matter
Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. If, on an energy diagram, the products have more stored energy than the reactants started with, the reaction is endothermic. You had to give the reaction energy.

Matter Anchor Chart Physical vs. Chemical Changes Science chemistry
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist.

Physical Changes of Matter YouTube
1. Intro to General Chemistry Physical & Chemical Changes Previous Topic Next Topic A physical change involves a change in the phases of matter, whereas a chemical change involves a change in chemical bonds. 1 concept Physical and Chemical Changes 1m 2 Comments Mark as completed Was this helpful? 51 2 example Physical & Chemical Changes Example 1

PPT Physical vs. Chemical Changes PowerPoint Presentation, free
This chemistry video tutorial explains the differences between physical vs chemical changes. Examples of physical changes include melting, freezing, evapora.
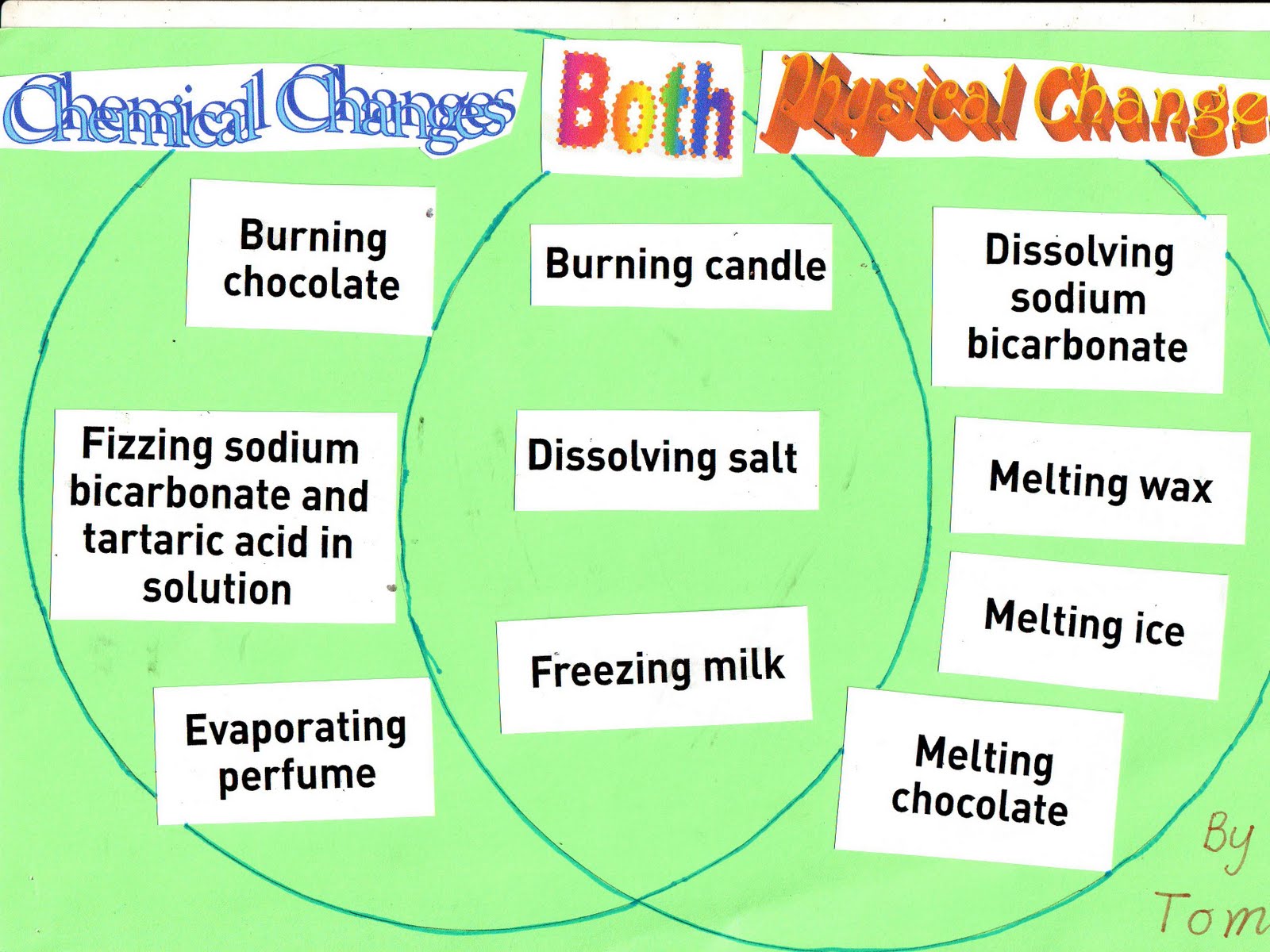
Change Detectives Tom 18
Updated on May 05, 2019 A physical change is a type of change in which the form of matter is altered but one substance is not transformed into another. The size or shape of matter may be changed, but no chemical reaction occurs. Physical changes are usually reversible.
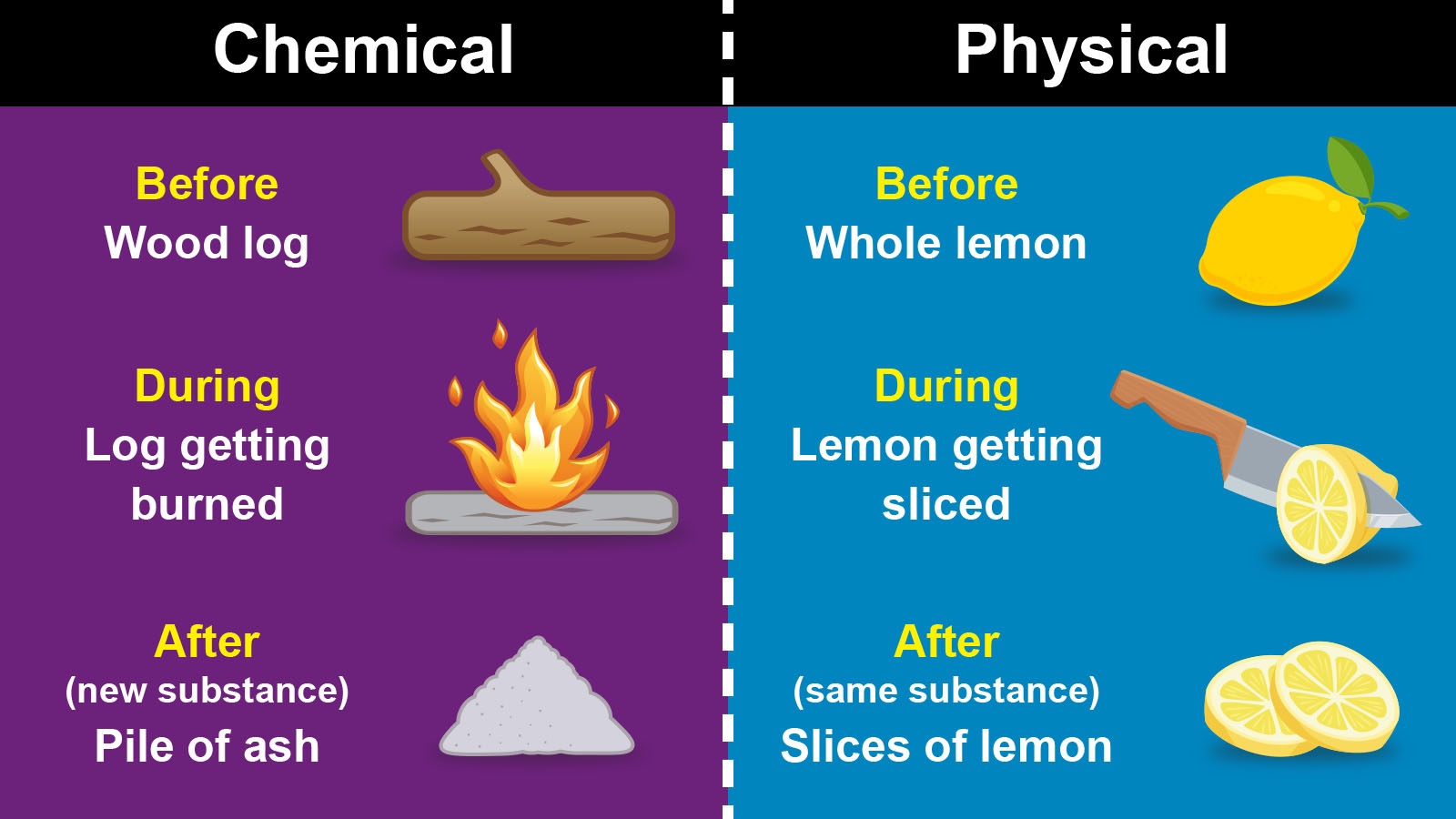
Main Difference Between a Chemical and Physical Change
51,901 Physical Change When matter changes its observable properties, we can say it has undergone a physical change. Physical change is a type of change where the physical properties of matter change. A change of state of matter, change in colour, odour, solubility, etc. all are examples of physical change.
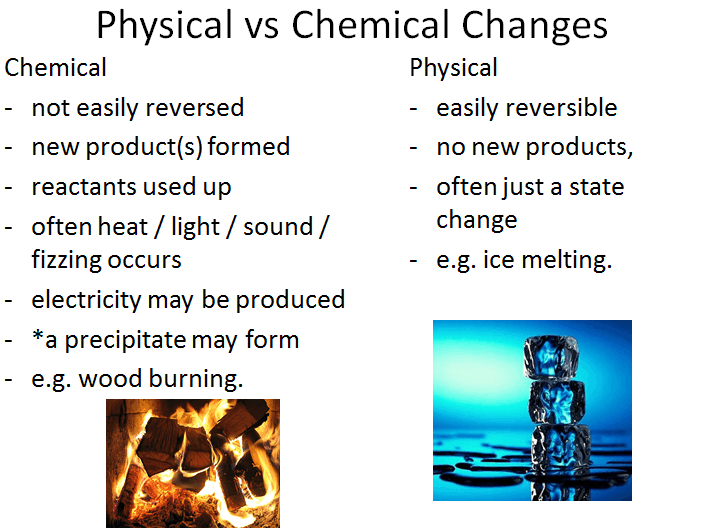
Science online What is the difference between the physical changes and
Physical Change Part of Science Physics Remove from My Bitesize Physical changes such as state change and dissolving are reversible, and there is no change in total mass when they.
Chemical Change Physical Change Diagram Chemistry Matter, PNG
However, physical changes can be exothermic or endothermic. The melting of an ice cube, which is endothermic, is a change in a physical property and not composition. Thus, it is a physical change. Change in Color. A change in color is also another characteristic of a chemical reaction taking place. For example, if one were to observe the.

PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation
Updated on January 24, 2020 Physical changes involve states of matter and energy. No new substance is created during a physical change, although the matter takes a different form. The size, shape, and color of matter may change. Physical changes occur when substances are mixed but don't chemically react. How to Identify a Physical Change
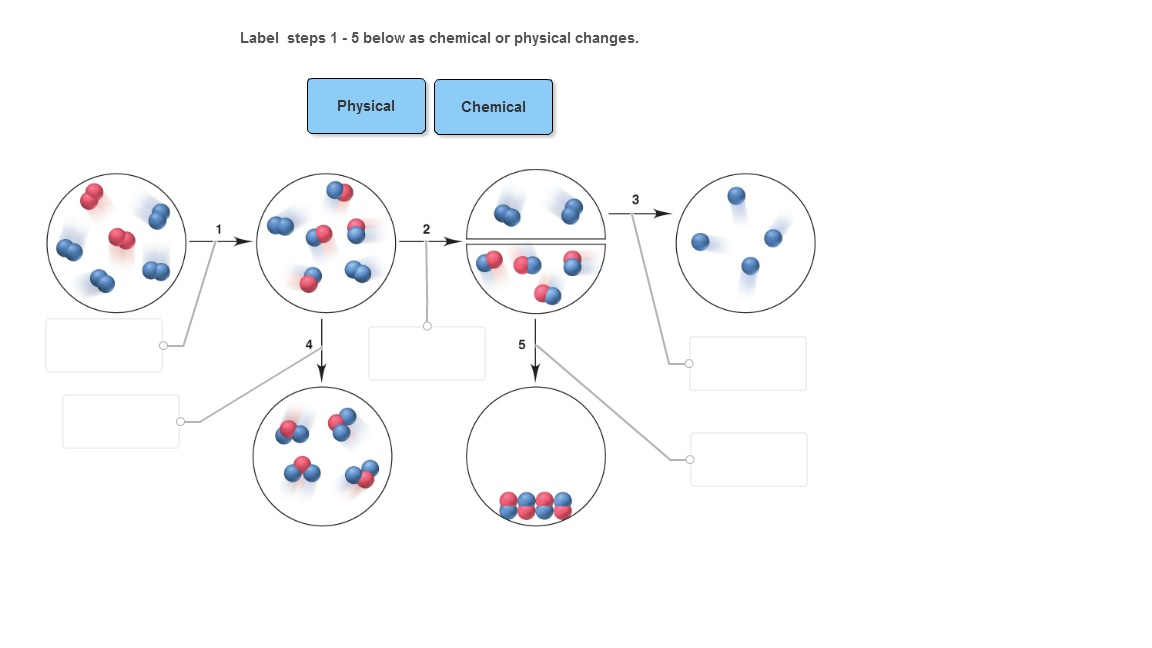
Solved Label steps 1 5 below as chemical or physical changes
Selected text level. Matter is capable of undergoing changes, which are classified as either physical or chemical. Physical changes in matter are often reversible: An ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. Chemical changes, on the other hand, are not reversible: A log burned in a fire.
Science online What is the difference between the physical changes and
Google Classroom About Transcript Physical and chemical processes can be classified by the changes occurring on the molecular level. In general, chemical processes involve changes in chemical bonds, while physical processes involve changes only in intermolecular forces.
