[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
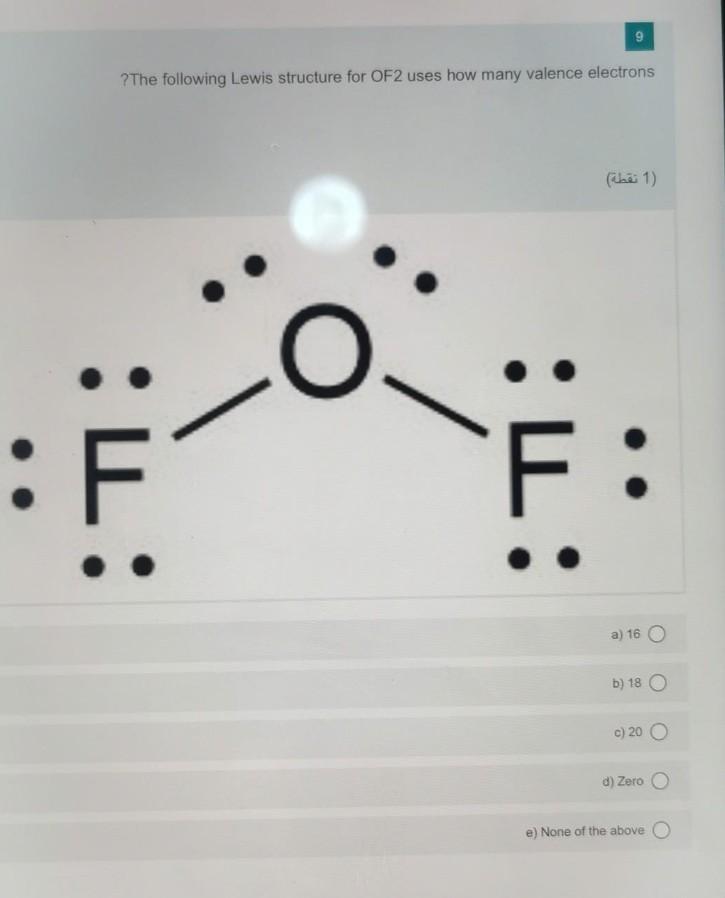
Solved 9 ?The following Lewis structure for OF2 uses how
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(N) = 5 −(0 + 8 2) = 0.

What is the Lewis dot structure of \ce{OF2}? Quizlet
How to Draw Lewis structure for OF2 Oxygen difluorideLewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Subs.

OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
The Lewis structure of OF2 molecule contains 16 non-bonding electrons i.e. 8 lone pairs. From the 8 lone pairs, 3 lone pairs are present on the fluorine atom and 2 lone pairs are present on the central atom oxygen. OF2 molecule details- How to draw lewis structure for OF2?
[Solved] What is the correct Lewis structure for oxygen difluoride
For \(\ce{OF2}\), we had 16 electrons remaining in Step 3, and we placed 12, leaving 4 to be placed on the central atom:. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons.

Draw the Lewis structures of the given molecules. Include lone pairs on
Lewis structure of OF2 contains two single bonds between the Oxygen (O) atom and each Fluorine (F) atom. The Oxygen atom (O) is at the center and it is surrounded by 2 Fluorine atoms (F). The Oxygen atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.

In this video we are going to determine the polarity of Oxygen
= 20 valence electrons Thus, there are 20 valence electrons available for OF2 that help the atoms to form bonds. OF2 Lewis Structure Now that we know the total number of valence electrons for OF2, we can make the Lewis dot structure of OF2.
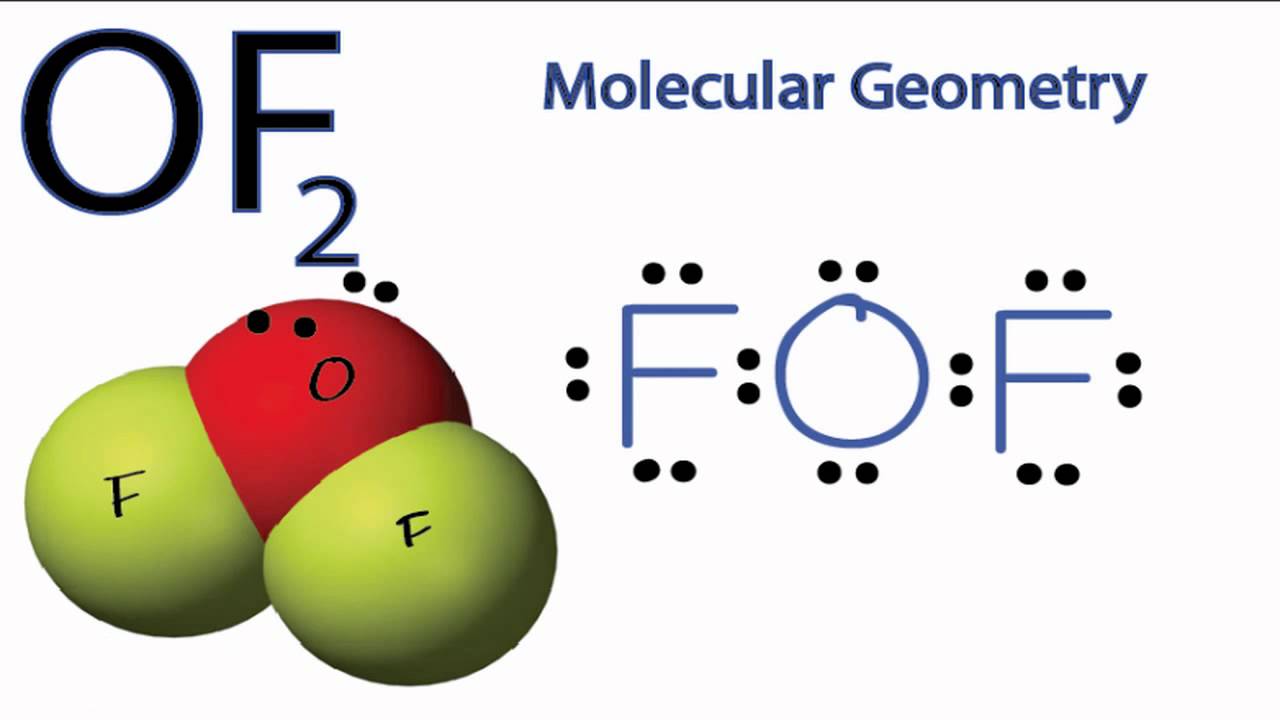
OF2 Molecular Geometry YouTube
OF2, aka Oxygen Difluoride, is a MOLECULAR compound.Oxygen brings 6 electrons, each Fluorine brings 7 electrons, that's 20 electrons total.That's enough for.

Answer please! In OF2, the total number of bond pairs and lone pairs of
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

What are the bond angles in the central atom of the following NSF, OF2
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons:. {OF2}\\ \phantom{+ }\textrm{O: 6 valence electrons/atom × 1 atom} \hspace{10px.
Solved 9 ?The following Lewis structure for OF2 uses how
To create the Lewis structure of OF2, follow these steps: 1. Counting Valence Electrons Begin by counting the total valence electrons in the molecule. In OF2, we have: Oxygen (O) - 6 valence electrons Fluorine (F) - 7 valence electrons each (2 atoms) Total valence electrons = 6 + 7*2 = 20 2. Placing the Least Electronegative Atom in the Center

Of2 Lewis Structure Of2 Lewis Structure Molecular Geometry
13K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the Lewis Dot Structure for Oxygen Difluoride, along with information about the compound including.
OF2 lewis structure, molecular geometry, hybridization and bond angle
-the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for O 2 ( Oxygen Difluoride) Oxygen difluoride (OF 2) isn't too tough of a Lewis structure since it only has single bonds. There are 20 valence electrons available for the Lewis structure for OF 2. So you'll be busy drawing some dots.

using VSEPR theory explain type of hybridization and draw the structure
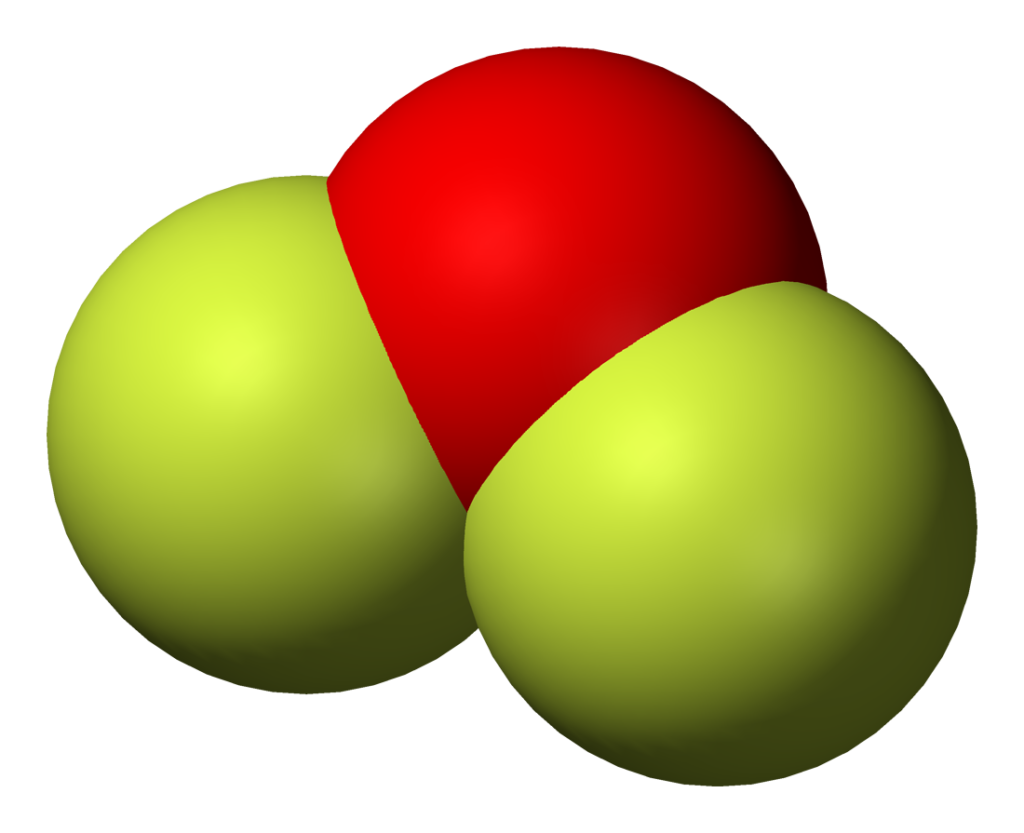
The information on this page is fact-checked. OF 2 Lewis structure. OF 2 (oxygen difluoride) has one oxygen atom and two fluorine atoms. In the OF 2 Lewis structure, there are two single bonds around the oxygen atom, with two fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the oxygen atom has two lone pairs.
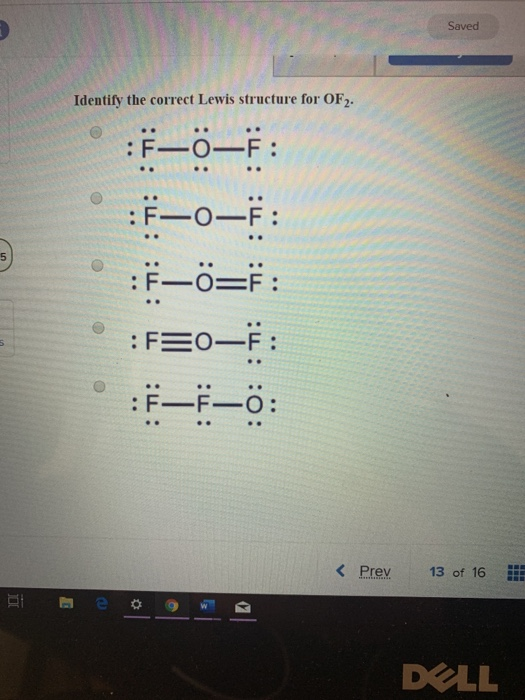
Solved Saved Identify the correct Lewis structure for OF2.
The Lewis structure indicates that each Cl Cl atom has three pairs of electrons that are not used in bonding (called lone pairs ) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond .
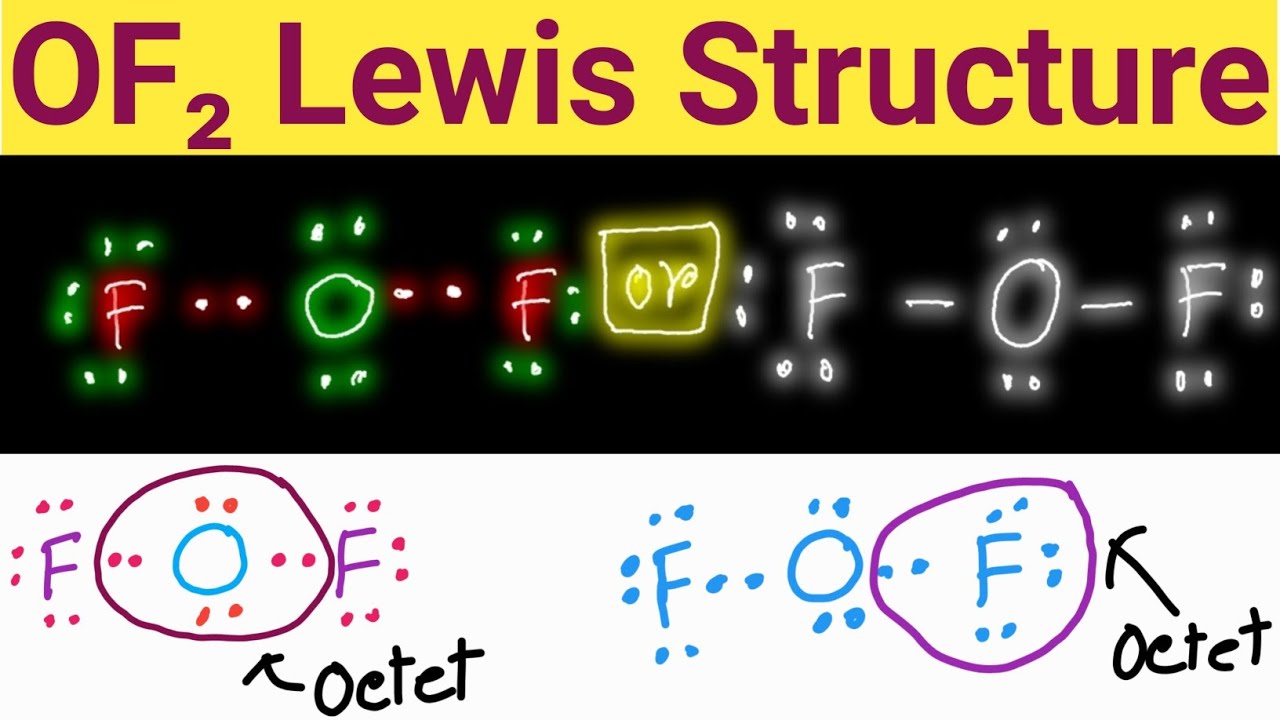
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
.more Hey Guys,In this video we are going to learn about the Lewis structure of OF2. It is a chemical formula for Oxygen Difluoride. This molecule is made up of on.
